Diamond is the hardest natural substance on Earth.
Why is diamond so strong?
Diamond is made up of a network of carbon atoms that each form four strong covalent bonds. The strong bonds take a lot of energy to break, so diamond has a high melting and boiling point.
This type of structure is called a giant covalent structure, as all the carbon atoms are bonded to each other by strong covalent bonds.
The strong covalent bonds also hold the atoms in a rigid lattice structure, which makes diamond very hard.
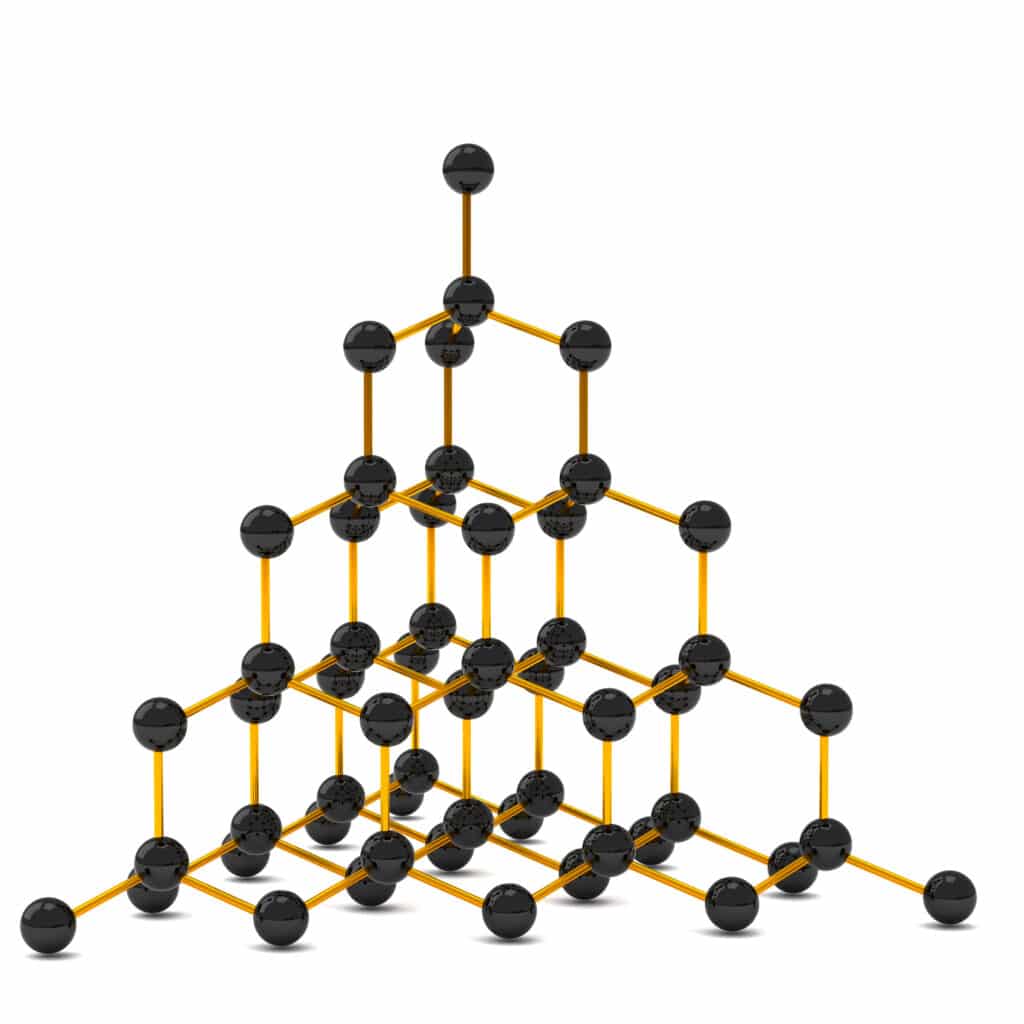
Uses of diamond
In cutting tools
Jewellery
Diamond does not conduct electricity as there are no free electrons.


Learn more about diamonds over on Live Science.

Last Updated on January 8, 2024 by Emma Vanstone


